Balanced Equation for the Decomposition of Aluminum Chloride
Mg s 2HBr aq -- MgBr2 aq H2 g b. When aluminium chloride decomposes then it gives aluminium and chlorine gas.
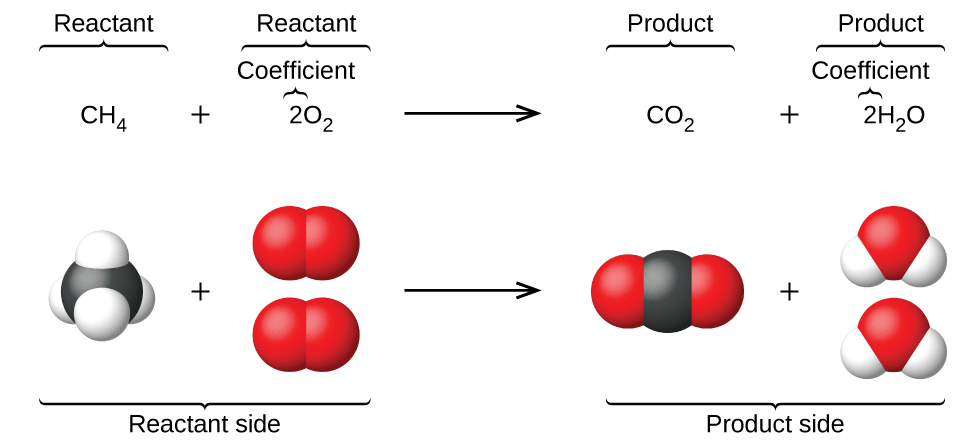
4 1 Writing And Balancing Chemical Equations Chemistry
On cooling the reaction is reversed and solid ammonium chloride reforms.
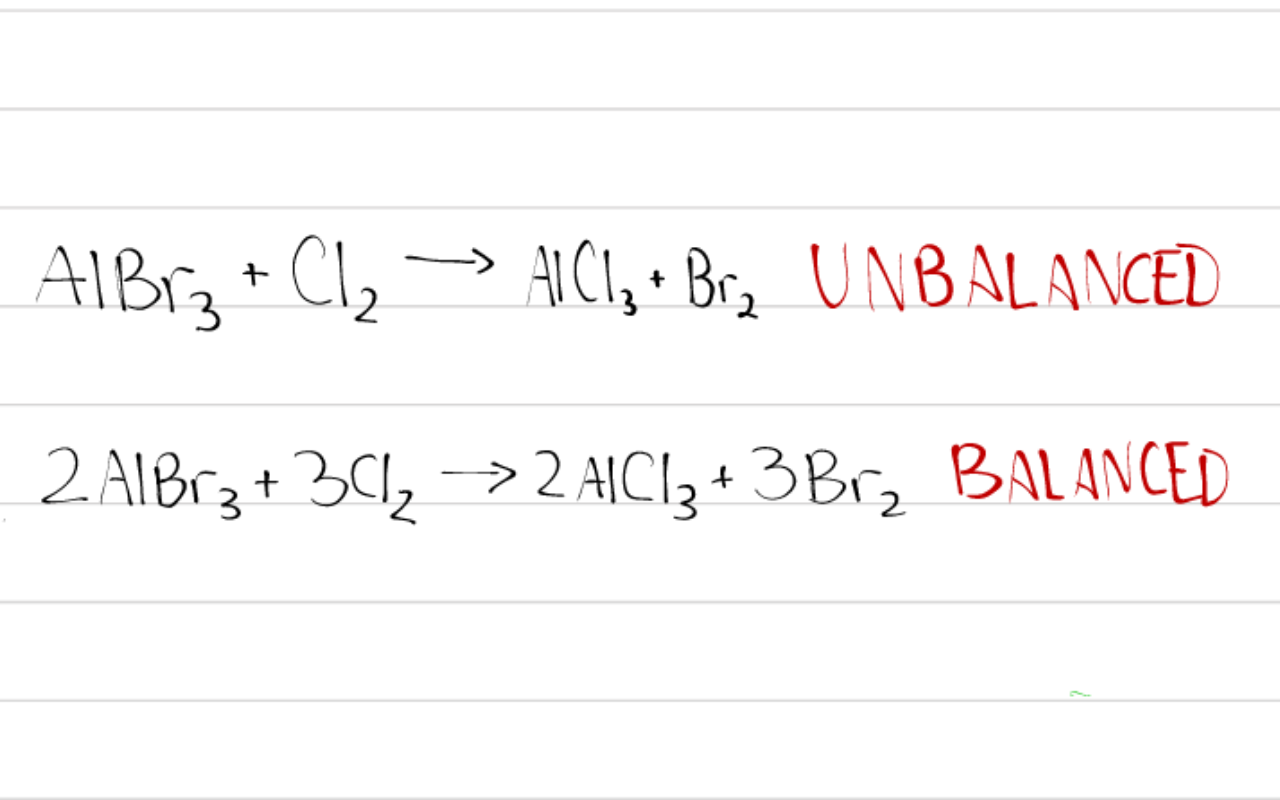
. Aluminium chlorate is decomposed to produce aluminium chloride and oxygen according to the equation. The equation for this reaction is. And today were going to be going over how to write an equation for the decomposition of aluminum chloride.
Phase symbols are optional. What is the balanced equation for aluminum chloride and copper. A balanced equation is an equation in which number of molecules on both left and right side are the same or equal.
4Fe 3O2 2Fe2O. B Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water. Phase symbols are optional.
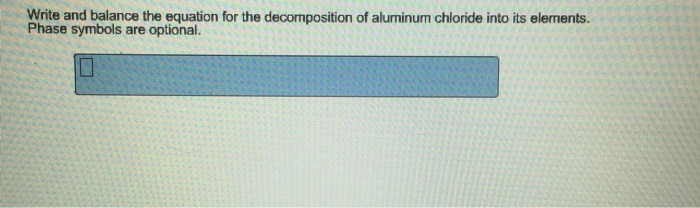
What is the balanced equation for aluminum plus hydrogen chloride equals aluminum chloride plus hydrogen. Write and balance the equation for the decomposition of aluminum chloride into its elements. Liquid aluminum chloride will decompose if an electrical current is.
Sal ammoniac is a name of the natural mineralogical form of ammonium chloride. The aluminum metal reacts with copper chloride ions that is sprinkled over the foil. Write and balance the equation for the decomposition of aluminum chloride into its elements.
Write and balance the equation for the decomposition of aluminum chloride into its elements. 3BaBr2 aq 2Na3PO4 aq -- Ba3 PO42 s 6NaBr aq d. Write a balanced equation to represent the electrolysis of molten sodium chloride.
Write and balance equation decomposition af aluminum chloride elements. Write and balance the equation for the decomposition of aluminum chloride into its elements. What volume of Cl 2 at STP is formed at the anode when 100 g of sodium is formed at the cathode.
3AgNO3 aq AlI3 aq -- 3AgI s Al NO33 aq Write the balanced chemical equation for the following reaction using lowest whole-number coefficient. 2Al 6HCl --. Decomposition in chemistry is the chemical separation of a compound.
2AlClO₃₃ 2AlCl₃ 9O₂. Phase symbols are optional. First you need the balanced equation N2 H2 NH3 we need it balanced N2 3H2 2NH3 now we have it balanced we have excess H2 so it doesnt play anything in the equation.
California State University - Los Angeles. What happens when aluminum reacts with copper II chloride. Write balanced equations for the reactions of aluminum with HClaq mathrmCl_2 and mathrmO_2.
This equation illustrates the use of coefficients to balance chemical equations. Write a balanced equation for the decomposition of aluminum chloride. Phase symbols are optional.
Apling Learning Write and balance the equation for the decomposition of aluminum chloride into its elements. We have ALCL-three ALCL3 which is the aluminum chloride. Previous oe Up View savudion Check Answer about us careers privacy policy fterma of use WHEN THE FLOWERS BLOOM SO WILL GREAT JOY IN YOUR LIFE.
That every 20 moles of aluminium chlorate is decomposed to produce 20 moles of aluminium chloride and 90 moles of oxygen. 1 mole N2 produces 2 moles NH3 351 moles N2 - 2351 moles NH3 we produce 702 moles of NH3 we need the molar mass of NH3 N 14 grams H 1 gram. What is the balanced equation for the electrolysis of aluminum chloride.
And then we draw an arrow to show what its breaking into. The molar mass of aluminum chloride is 13333 gmole. Phase symbols are optional.
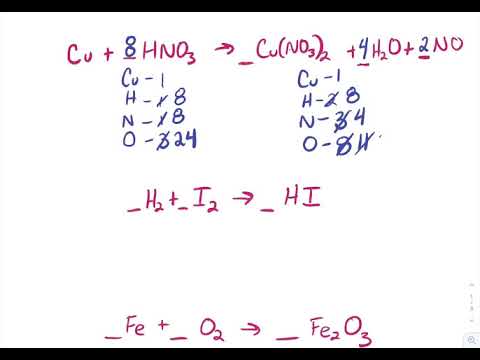
Its going to breakdown into aluminum plus chlorine which CL-two CL2. 2 Al 3 Cl2. _____ClO 3-1 O 2 _____Cl-1 Example.
Ca ClO32 s -- CaCl2 s 3O2 g c. Potassium Chlorate Potassium Chlorate Oxygen Potassium Chloride 2 KClO 3 3 O 2 2 KCl Example. So we start by writing the different substances.
Write and balance the equation for the decomposition of aluminum chloride into its elements. Phase symbols are optional. Phase symbols are optional.
Aluminum Carbonate Carbon Dioxide Aluminum Oxide Al 2 CO 3 3 3 CO 2 Al 2 O 3 Chlorates. When heated it decomposes into oxygen gas and a chloride. Al CuCl2 AlCl3 Cu Chemical Equation Balancer.
Fhasc symbols are optional.

Write The Balanced Equations For The Following Reactions A Iron Iii Oxide With Nitric Acid B Homeworklib

Al Hcl Alcl3 H2 Balanced Equation Aluminum Hydrochloric Acid Yield Aluminum Chloride Hydrogen Youtube

Alcl3 Al Cl2 Balanced Chemical Equation
5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts

5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts

How To Balance Al Cucl2 Alcl3 Cu Aluminum Copper Ii Chloride Youtube

What Is The Equation For The Decomposition Of Aluminium Chloride Youtube
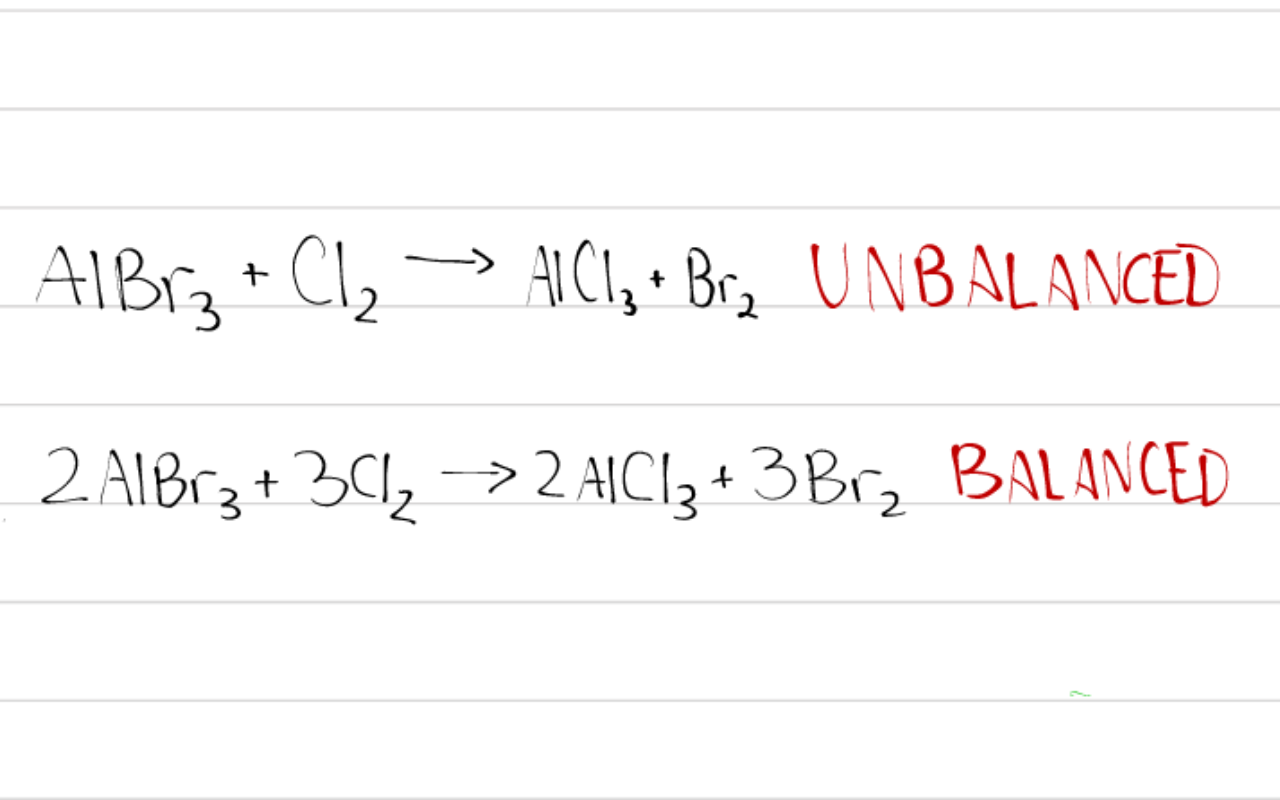
How Do You Write The Equation For This Reaction Aluminum Bromide And Chlorine Gas React To Form Aluminum Chloride And Bromine Gas Socratic

How To Balance Al Hcl Alcl3 H2 Youtube

What Is The Equation For The Decomposition Of Aluminum Chloride Chemistry Help Youtube

Balancing Bacl2 Al2 So4 3 Baso4 Alcl3 Youtube

How To Balance Alcl3 Al Cl2 Aluminum Chloride Decomposition By Electrolysis Youtube

Solved Homework Exercises 1 Write The Balanced Chemical Chegg Com
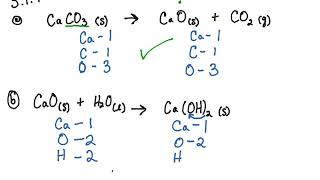
5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts
Solved Write And Balance The Equation For The Decomposition Chegg Com

Predicting Products Of Chemical Reactions Chemical Reactions Chemistry Education Teaching Chemistry
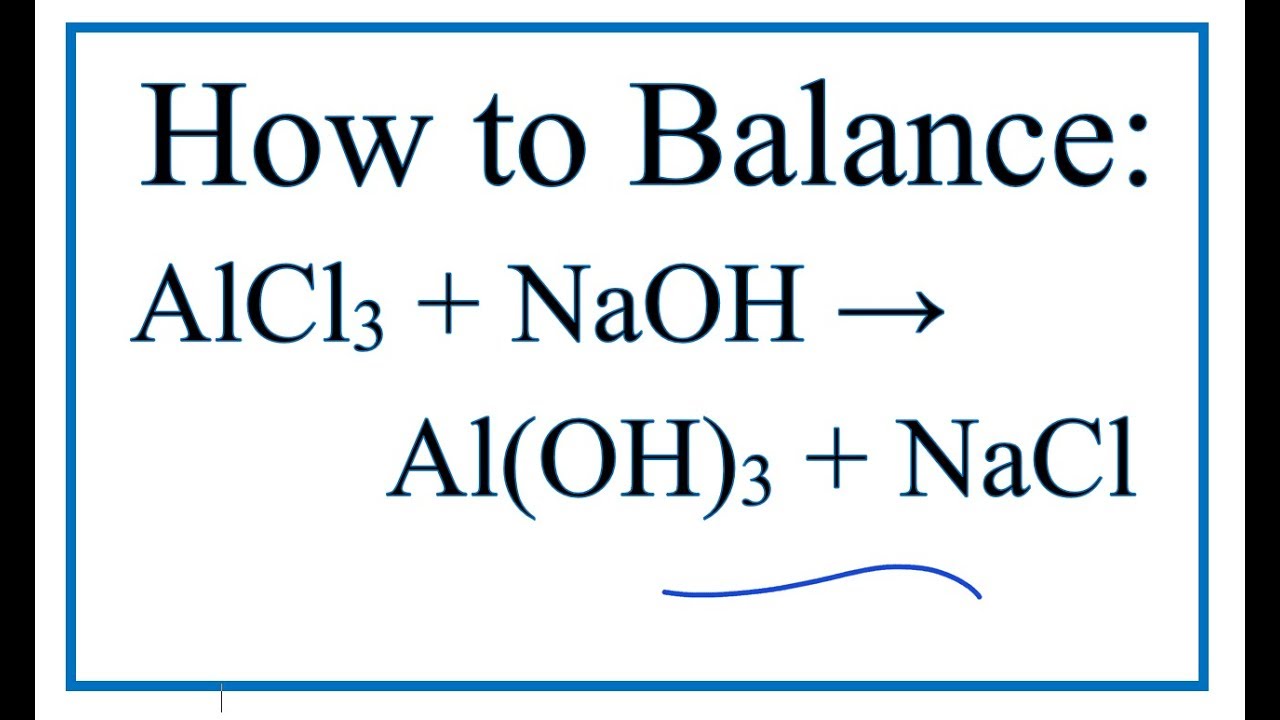
How To Balance Alcl3 Naoh Al Oh 3 Nacl Aluminum Chloride Sodium Hydroxide Youtube


Comments
Post a Comment