Describe the Bonding in Metals Using Electron Sea Model
Academiaedu is a platform for academics to share research papers. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.

Metallic Bonding Metallic Bond N N N Occurs
Mercury is a liquid at room temperature and the alkali metals melt below 200 C.

. Its monatomic form H is the most abundant chemical substance in the Universe constituting. Their multiple industrial domestic agricultural medical and technological applications have led to their wide distribution in the environment. Thus neglecting non-local and local pd-mixing it rigorously refers to the local 1s 3d quadrupole transition.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. XANES spectra can be further split into the pre-edge region and the main edge. Several post-transition metals also have low melting points whereas the transition metals melt at temperatures above 1000 C.
Question 11 Calculate the molecular mass of the following. Using the symmetric configuration in the SFA several interactions have emerged as significant in cohesion between Mfps Fig. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.
These differences reflect differences in strengths of metallic bonding among the metals. By using our website you can be sure to have your personal information secured. The melting points of the metals vary widely.
Most substances on Earth are compounds containing multiple elementsChemical bonding describes how these atoms attach with each. This can easily be observed in a. 1 bidentate intercatechol H-bonding is robust in aqueous solution Ahn et al 2014.
We give anonymity and confidentiality a first priority when it comes to dealing with clients personal information. A model of a water molecule showing the bonds between the hydrogen and oxygen. USGSAll other elements are less than 1.
I H2O ii CO2 iii CH4 Question 12 Calculate the mass per cent of different elements present in sodium sulphate Na2SO4. Question 13 Determine the empirical formula of an oxide of iron which has 699 iron and 301 dioxygen by mass. The K pre-edge for 3d transition metals corresponds to the promotion of an electron from the 1s orbital K-shell into a 3d orbital M-shell.
Raising concerns over their potential effects on human health and the environment. Water is the chemical substance with chemical formula H 2 O. Heavy metals are naturally occurring elements that have a high atomic weight and a density at least 5 times greater than that of water.
Question 14 Calculate the amount of carbon dioxide. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. 2 cation-π interactions between Lys and Dopa Phe Tyr or Trp which are surprisingly cohesive in the Mfps Lu et al 2013a.
For historical reasons ammonia is named ammine in the nomenclature. Eight Most Abundant Elements in the Earths Continental Crust by weight source. We do not at any time disclose clients personal information or credentials to third parties.
Ammonia can act as a ligand in transition metal complexesIt is a pure σ-donor in the middle of the spectrochemical series and shows intermediate hardsoft behaviour see also ECW modelIts relative donor strength toward a series of acids versus other Lewis bases can be illustrated by C-B plots.

Section 7 4 Section 7 4 Metallic Bonds And The Properties Of Metals Describe A Metallic Bond Relate The Electron Sea Model To The Physical Properties Ppt Download
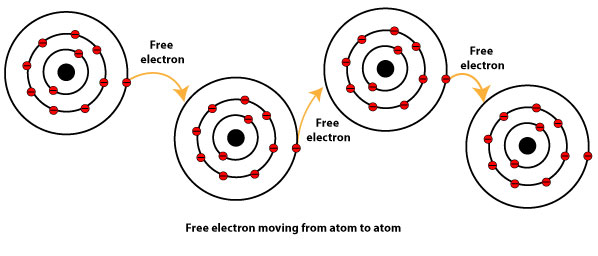
What Is Metallic Bonding Using The Electron Sea Model To Explain It

Metallic Bonding 7 3 Electron Sea Model The Electron Sea Model Proposes That All The Metal Atoms In A Metallic Solid Contribute Their Valence Electrons Ppt Download

Learn About Electron Sea Model Chegg Com
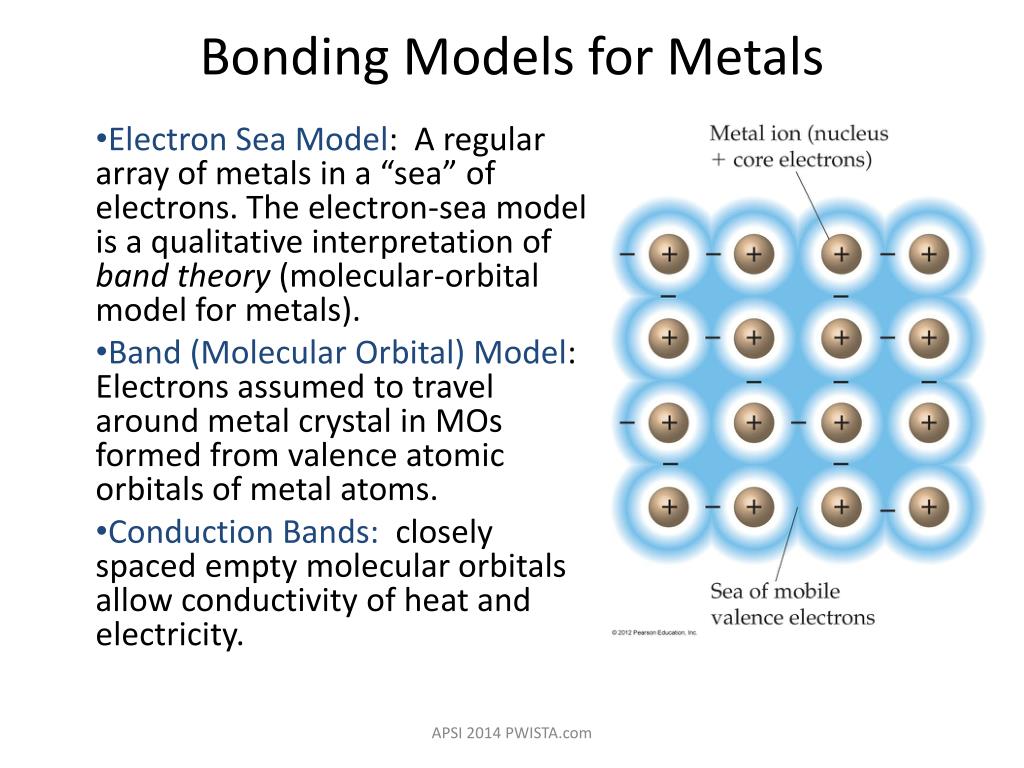
Ppt Metallic Bonding Alloys Semiconductors Powerpoint Presentation Id 1586023

Metallic Bonding And The Electron Sea Model Electrical Conductivity Basic Introduction Youtube
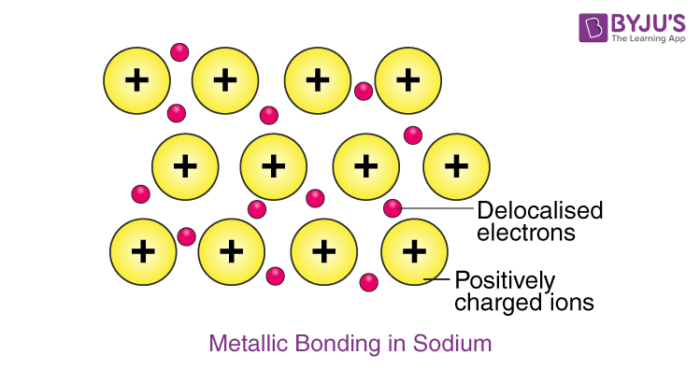
Metallic Bonds Properties Examples Explanation Of Metallic Bonds
Properties Of Metallic Bonding Ppt Download
What Is The Electron Sea Model Of Metallic Bonding Quora
What Are Some Ways To Describe Metallic Bonding Quora
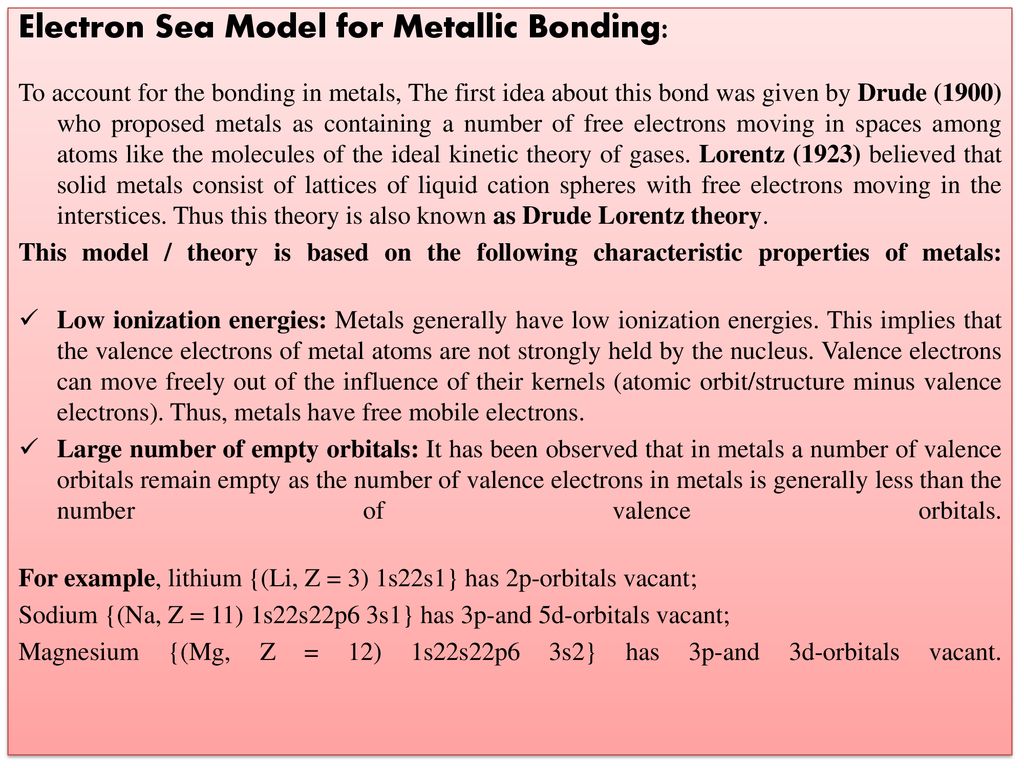
Title Metallic Bond Dr Ppt Download

Metallic Bonding Definition And Properties
Properties Of Metallic Bonding Ppt Download

Metallic Bonding 7 3 Electron Sea Model The Electron Sea Model Proposes That All The Metal Atoms In A Metallic Solid Contribute Their Valence Electrons Ppt Download

Bonding In Metals The Electron Sea Model Introduction To Chemistry
Metallic Bonding Properties Tutorial Now With Animations The Crash Chemistry Academy Youtube
12 4 Bonding In Metals And Semiconductors Chemistry Libretexts


Comments
Post a Comment